Spdf Periodic Table / Spdf Table In Chemistry | Decoration Jacques Garcia : Group 2 elements occur directly to the right of group 1 elements.
Spdf Periodic Table / Spdf Table In Chemistry | Decoration Jacques Garcia : Group 2 elements occur directly to the right of group 1 elements.. Each block is named after its characteristic orbital: Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g). By spdf configuration, he meant orbital configuration. Two types of s block elements are possible i.e. The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells.
Electrons exist around the nucleus of an atom in discrete, specific orbits. The highest energy level (valence shell) contains only 1 electron in an s subshell. The s , p , d , and f blocks are illustrated below. Jun 14, 2015 · use the element blocks of the periodic table to find the highest electron orbital. For example, all the elements of group 2 have an electron configuration of [e] ns 2 (where [e] is an inert gas configuration), and have notable similarities in their chemical properties.
 IB Chemistry on Quantum Numbers and Electronic Configuration from image.slidesharecdn.com
IB Chemistry on Quantum Numbers and Electronic Configuration from image.slidesharecdn.com
Now the basic of this concept is from very fundamental quantum chemistry formulation but i don't think you need to know that now (it is usually taught in 4th year of ug or 1st year of graduate study in engineering discipline but i am not sure about pure science discipline). Levels, sublevels, orbitals, and electrons!!! Electrons can not just exist at any distance from the nucleus. By spdf configuration, he meant orbital configuration. The term appears to have been first used by charles janet. The s , p , d , and f blocks are illustrated below. Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g). The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal qu.
Two types of s block elements are possible i.e.
Learn what element blocks are and their properties and characteristics. The term appears to have been first used by charles janet. By spdf configuration, he meant orbital configuration. Each block is named after its characteristic orbital: Group 2 elements occur directly to the right of group 1 elements. Group 1 elements occur at the beginning of a new row (period) of the periodic table. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal qu. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. Levels, sublevels, orbitals, and electrons!!! Jun 14, 2015 · use the element blocks of the periodic table to find the highest electron orbital. Electrons can not just exist at any distance from the nucleus. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in.
Learn what element blocks are and their properties and characteristics. The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells. Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g). For example, all the elements of group 2 have an electron configuration of [e] ns 2 (where [e] is an inert gas configuration), and have notable similarities in their chemical properties. Each block is named after its characteristic orbital:
.svg/799px-Periodic_table_blocks_spdf_(32_column).svg.png) File:Periodic table blocks spdf (32 column).svg ... from upload.wikimedia.org
File:Periodic table blocks spdf (32 column).svg ... from upload.wikimedia.org
Group 2 elements occur directly to the right of group 1 elements. The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occurs. As shown in table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes. The highest energy level (valence shell) contains only 1 electron in an s subshell. Now the basic of this concept is from very fundamental quantum chemistry formulation but i don't think you need to know that now (it is usually taught in 4th year of ug or 1st year of graduate study in engineering discipline but i am not sure about pure science discipline). Learn what element blocks are and their properties and characteristics. Each block is named after its characteristic orbital: A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in.
Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g).
The form of the periodic table is closely related to the electron configuration of the atoms of the elements. The s , p , d , and f blocks are illustrated below. Each block is named after its characteristic orbital: Group 2 elements occur directly to the right of group 1 elements. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. Levels, sublevels, orbitals, and electrons!!! Jun 14, 2015 · use the element blocks of the periodic table to find the highest electron orbital. The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells. Learn what element blocks are and their properties and characteristics. The highest energy level (valence shell) contains only 1 electron in an s subshell. By spdf configuration, he meant orbital configuration. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occurs.
Each block is named after its characteristic orbital: Now the basic of this concept is from very fundamental quantum chemistry formulation but i don't think you need to know that now (it is usually taught in 4th year of ug or 1st year of graduate study in engineering discipline but i am not sure about pure science discipline). The highest energy level (valence shell) contains only 1 electron in an s subshell. The term appears to have been first used by charles janet. Levels, sublevels, orbitals, and electrons!!!
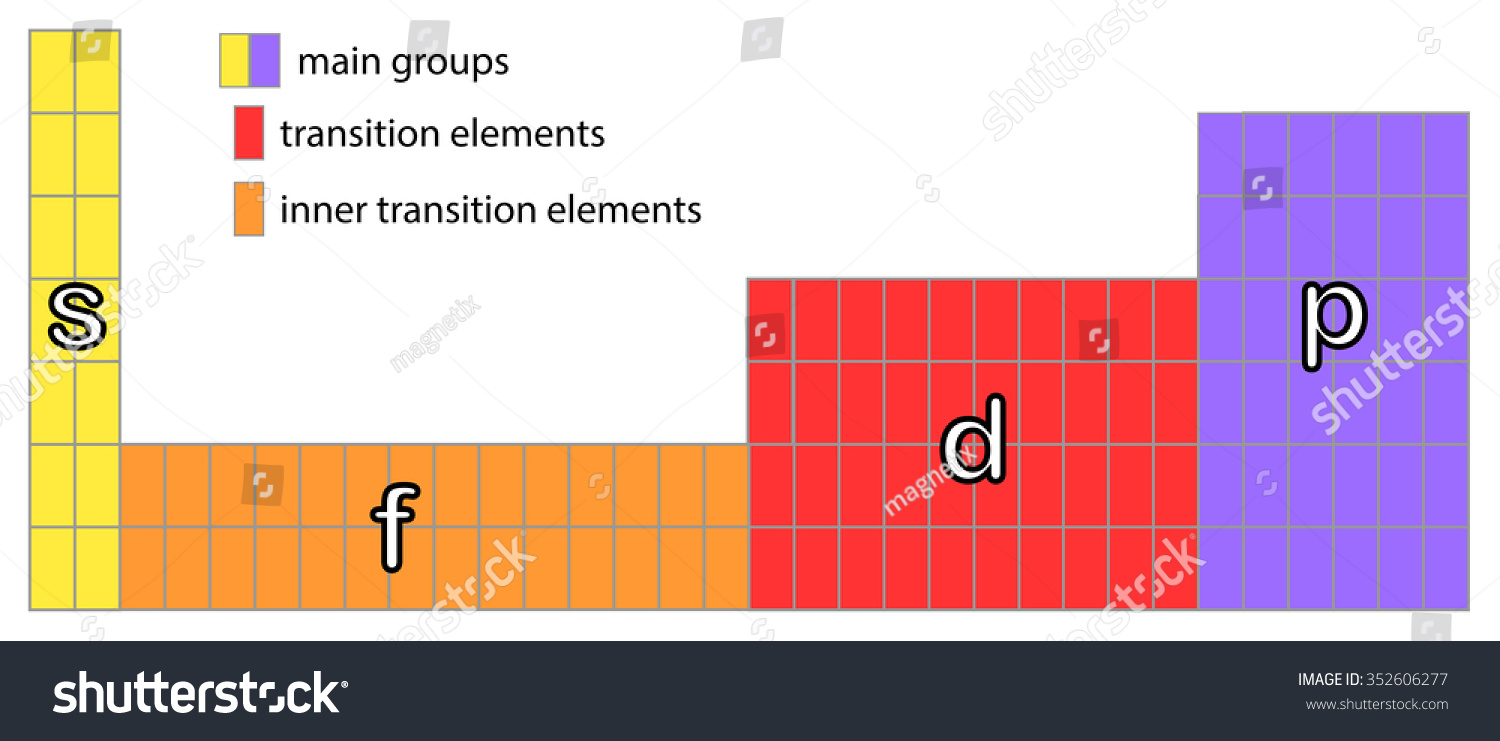 Spdf Table Of Elements | Decoration Jacques Garcia from image.shutterstock.com
Spdf Table Of Elements | Decoration Jacques Garcia from image.shutterstock.com
The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. By spdf configuration, he meant orbital configuration. Two types of s block elements are possible i.e. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. As shown in table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes. The form of the periodic table is closely related to the electron configuration of the atoms of the elements. Jun 14, 2015 · use the element blocks of the periodic table to find the highest electron orbital. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in.
Electrons exist around the nucleus of an atom in discrete, specific orbits.
Two types of s block elements are possible i.e. Group 1 elements occur at the beginning of a new row (period) of the periodic table. The term appears to have been first used by charles janet. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. Group 2 elements occur directly to the right of group 1 elements. The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occurs. The highest energy level (valence shell) contains only 1 electron in an s subshell. Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g). For example, all the elements of group 2 have an electron configuration of [e] ns 2 (where [e] is an inert gas configuration), and have notable similarities in their chemical properties. The form of the periodic table is closely related to the electron configuration of the atoms of the elements. The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. Learn what element blocks are and their properties and characteristics.
Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled spd The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal qu.
As shown in table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. Group 1 elements occur at the beginning of a new row (period) of the periodic table. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. Group 2 elements occur directly to the right of group 1 elements.
Source: showme0-9071.kxcdn.com
Group 1 elements occur at the beginning of a new row (period) of the periodic table. Each block is named after its characteristic orbital: Electrons can not just exist at any distance from the nucleus. The s , p , d , and f blocks are illustrated below. Jun 14, 2015 · use the element blocks of the periodic table to find the highest electron orbital.
Source: lh5.googleusercontent.com
Learn what element blocks are and their properties and characteristics. The s , p , d , and f blocks are illustrated below. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal qu. A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g).
Source: i.pinimg.com
As shown in table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes. By spdf configuration, he meant orbital configuration. Electrons exist around the nucleus of an atom in discrete, specific orbits. The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occurs.
Source: lh3.googleusercontent.com
The s , p , d , and f blocks are illustrated below. Group 1 elements occur at the beginning of a new row (period) of the periodic table. As shown in table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes. The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells. The term appears to have been first used by charles janet.
Source: i.ytimg.com
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The labels s, p, d and f blocks of the periodic table refer to the subshell that is being filled with electrons. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal qu. The term appears to have been first used by charles janet. The form of the periodic table is closely related to the electron configuration of the atoms of the elements.
Source: www.assignmentpoint.com
Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g). By spdf configuration, he meant orbital configuration. Each block is named after its characteristic orbital: Learn what element blocks are and their properties and characteristics. The term appears to have been first used by charles janet.
Source: staff.concord.org
The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. By spdf configuration, he meant orbital configuration. As shown in table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes. Each block is named after its characteristic orbital:
Source: www.thoughtco.com
Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled. The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal qu. The figure also illustrates how the d sublevel is always one principal level behind the period in which that sublevel occurs. Group 2 elements occur directly to the right of group 1 elements. Levels, sublevels, orbitals, and electrons!!!
Source: www.boyertownasd.org
Two types of s block elements are possible i.e.
Source: upload.wikimedia.org
Levels, sublevels, orbitals, and electrons!!!
Source: preparatorychemistry.com
Now the basic of this concept is from very fundamental quantum chemistry formulation but i don't think you need to know that now (it is usually taught in 4th year of ug or 1st year of graduate study in engineering discipline but i am not sure about pure science discipline).
Source: lh5.googleusercontent.com
Levels, sublevels, orbitals, and electrons!!!
Source: lh5.googleusercontent.com
Jun 14, 2015 · use the element blocks of the periodic table to find the highest electron orbital.
Source: www.thoughtco.com
Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g).
Source: www.thoughtco.com
As shown in table 1, the s subshell has one lobe, the p subshell has three lobes, the d subshell has five lobes, and the f subshell has seven lobes.
Source: ibalchemy.com
By spdf configuration, he meant orbital configuration.
Source: media.cheggcdn.com
The term appears to have been first used by charles janet.
Source: www.coursehero.com
Jun 14, 2015 · use the element blocks of the periodic table to find the highest electron orbital.
Source: i.ytimg.com
Apr 05, 2020 · the periodic table of elements can be organized by blocks (s, p, d, f, g).
Source: i.pinimg.com
The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal qu.
Source: i.ytimg.com
The highest energy level (valence shell) contains only 1 electron in an s subshell.
Source: i.pinimg.com
Each block is named after its characteristic orbital:
Source: i.pinimg.com
The highest energy level (valence shell) contains only 1 electron in an s subshell.
Source: lh3.googleusercontent.com
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in.
Source: upload.wikimedia.org
Jun 14, 2015 · use the element blocks of the periodic table to find the highest electron orbital.
Source: upload.wikimedia.org
The number of possible values is the number of lobes (orbitals) there are in the s, p, d, and f subshells.
Source: www.coscinecreative.com
Electrons can not just exist at any distance from the nucleus.
Source: www.thoughtco.com
Group 2 elements occur directly to the right of group 1 elements.
Source: i.ytimg.com
The block names (s, p, d, and f) are derived from the spectroscopic notation for the value of an electron's azimuthal qu.
Source: i.ytimg.com
For example, all the elements of group 2 have an electron configuration of [e] ns 2 (where [e] is an inert gas configuration), and have notable similarities in their chemical properties.
Source: www2.dawsoncollege.qc.ca
By spdf configuration, he meant orbital configuration.
Source: revise.im
For example, all the elements of group 2 have an electron configuration of [e] ns 2 (where [e] is an inert gas configuration), and have notable similarities in their chemical properties.
Source: lh5.googleusercontent.com
Levels, sublevels, orbitals, and electrons!!!